Resonance Form is very abstract, so it is difficult to explain in words.
You have to feel it with your body and understand it with your mind, not your head.
When expressing a molecule, it is difficult to describe the structure with one expression method, so it is possible to draw a different structure by pi bonds and unshared electron pairs while contributing to the final structure with a resonance structure.
Examples are acetate ion and benzene.

The method of describing the resonance structure conclusively tells us this 1.5 bond structure.
In the case of the acetate ion, it shows two resonance structures.

ou can show two resonance structures like this.
The resonance structure cannot express the line-coupled structure we use.
Just because these two resonance structures are shown, you should think that they do not exist separately, but are one structure with both characteristics.
Therefore, it was expressed as a structure of 1.5 bonds.
The same is true for the structure of benzene.

It looks like you've seen it a lot in chemistry class in middle school and high school.
You may have learned in chemistry class that benzene has a single bond and a double bond, but it does not.
There are also rules in this resonance structure.
1. The resonance structure is an imaginary structure.
As I said, it has a single structure that doesn't change, and it doesn't change the bond and structure by itself.
2. Only the arrangement of phi electrons or unshared electrons is different.
Acetate ion You can check the picture above.
3. Different resonance structures do not necessarily have to be equivalent in any compound.
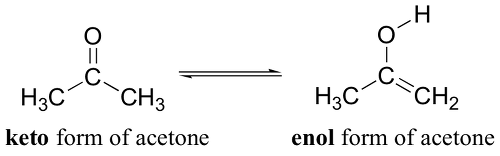
Please refer to the acetone enolate.
4. Follow the valence rules.
Lewis structure, octet rule, etc. are applied.
5. Resonant hybrids are more stable than any resonant structure.
Resonance hybridizes, and electrons disperse a large portion of the molecule and become close to several nuclei and become stable.

(Image source: www.chemthes.com/icon_2/21.gif
2 In 4-pentanedione, it has an unshared electron pair and a negative formal charge next to the left and right C=O double bonds.
If you give one, you can think of it as having one, and to do this, you only need to have a different double bond or an unshared pair of electrons.
Therefore, it is possible to draw three resonance structures, and I would like to inform you that the higher the number of resonance structures, the higher the acidic value.
In addition, it may be confused with isomers, but isomers are different.
The difference between isomer and resonance structure.
If the resonance structure changes only the electron arrangement due to the movement of the pie electrons,
An isomer is a change in the bond structure of an atom, so the resonant structure and the isomer have a different nature.
'과학 > 유기화학' 카테고리의 다른 글
| Covalent bond, dipole moment and formal charge (0) | 2021.02.14 |
|---|---|
| Hybrid orbital function and molecular orbital function, structural formula (0) | 2021.01.31 |
| LD50, national health indicator (0) | 2020.08.16 |
| Introduction to organic chemistry, history and hybrid orbital functions (0) | 2020.07.12 |
| 알켄의 수산화 반응, 환원 산화, 라디칼 첨가 (0) | 2016.05.23 |