Prior to the previous description, the previous post explained the shape of organic molecules and the bonding using hybrid orbitals.
Before explaining electronegativity and covalent bonds, briefly describing each bond is as follows.
1. A nonpolar covalent bond is a bond between atoms with similar electronegativity.
2. A polar covalent bond is a bond where the electronegativity difference between each atom is less than 2.
3. Ionic bonds are bonds with an electronegativity difference greater than 2 as opposed to polar covalent bonds.
For example, C-H (carbon-hydrogen bond) is relatively non-polar, and C-O (carbon-oxygen bond) and C-X (atom and carbon bond with a greater electronegativity) are polar.
In the C-H bond, C and carbon are partially positively charged, and on the contrary, in H and hydrogen, they are positioned as electronegative atoms with relatively negative charges.
Through this, the inductive effect mentioned in the first post is exerted.
Introduction to organic chemistry, history and hybrid orbital functions
You can think of organic chemistry as a study that shows curiosity about organic compounds in living organisms. Most organic compounds contain carbon, and as an example, th..
biostudy.tistory.com
This inducing effect is that electrons in the sigma bond simply move according to the electronegativity of adjacent atoms.
In other words, positively charged metals such as magnesium induce and inductively push electrons, and atoms of negatively charged nonmetals such as oxygen attract electrons in reverse.
There is something that shows this in a visual way, and that is the Electrostatic Potential Map.
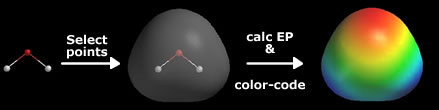
For example, I took H2O and water. Oxygen has a partial negative charge and shows red color, and hydrogen has a partial positive charge and shows blue color.
In addition, water has a dipole moment and a dipole moment as a polar covalent bond.
The larger the polarity difference, the larger the dipole value.
This dipole moment μ (greek letter, mu (which is when you use mutorent, utorent)) is the value of Q, which is the charge at the end of the dipole of the molecule, and the distance r between the two charges.
μ=Q*r and the unit is D(debyes), 1D=3.336*10^-30 C*m
If you find the isolated electron pair first, it is easy to calculate the dipole moment, so you can refer to it.
Find the lone pair first, then check the electronegativity from the middle atom.
In addition, there is a formal transfer for the double-pole moment and the polar bond, which is a very similar concept to the electrostatic potential map.
It is the concept that molecules do not have actual ionic charges, but are shared with each other and move.
Take an example with CH3SOCH3.
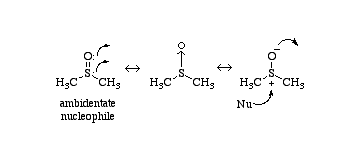
www.chemthes.com/icon_2/264.gif)
Where S, sulfur has 6 valence electrons, 6 bonded electrons, and 2 unshared electrons.
The formula to find the formal charge is the number of valence electrons-(number of bonded electrons/2)-non-covalent transfer number.
6-6/2-2=+1
Oxygen is 6,2,6 so it has -1
In simple terms, losing an electron is +1 (since the electron is -1, you can think of -(-1)
So, you only have to know how many you had, but how many were changed.
if you wanna read to korean,
공유 결합, 이중극자 모멘트와 형식전하
앞서 설명하기 전에 이 전 포스트까지로 유기 분자의 모양새 설명과 혼성 궤도함수를 이용한 결합에 대해 설명하였다 전기 음성도와 공유 결합에 설명하기 전 간단히 각 결합에 대해 설명하자
biostudy.tistory.com
'과학 > 유기화학' 카테고리의 다른 글
| Molecular resonance structure and resonance hybrid (0) | 2021.02.14 |
|---|---|
| Hybrid orbital function and molecular orbital function, structural formula (0) | 2021.01.31 |
| LD50, national health indicator (0) | 2020.08.16 |
| Introduction to organic chemistry, history and hybrid orbital functions (0) | 2020.07.12 |
| 알켄의 수산화 반응, 환원 산화, 라디칼 첨가 (0) | 2016.05.23 |