Biomolecules tend to form polymers from small units to large units when forming polymers.
Amino acids, monosaccharides, and nucleotides are examples.
From Monomers to Polymers, the benefit of this is that the benefits to cells are achieved by arranging from the raw material limitations of the monomers, which means that the chemical bonds between each unit encode information in a stable form.
However, the lipid is characterized by having a large amount of binding, but not forming a polymer, which is a polymer.

Protein is a polymer of amino acids, and 20 kinds of amino acids have different types of protein in order, and polypeptides are formed by amino acids.
H20, shows a dehydration condensation reaction in which water escapes, and it is called a peptide bond
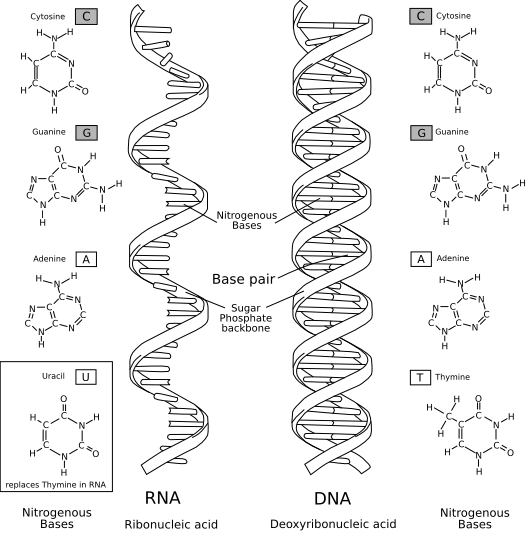
Nucleic acids synthesize proteins from ribosomes and bind polynucleotides to form DNA and RNA
It is composed of 4 nucleotides, A, T, C, G, (U).
Nucleotides are characterized by less variable structures and more regularity than proteins.
In addition, it is characterized in that it can be decomposed by alkali hydrolysis while performing phosphoric acid diester bonding and phosphodiest bond.
The difference between DNA and RNA is that DNA is the sugar of RNA minus 2'oxygen.
Therefore, the name is called 2'-deoxy-ribose, and to solve the meaning, it is ribose with 2 prime oxygen removed.
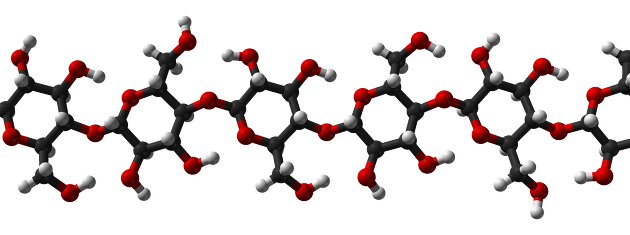
Polysaccharides are a combination of monosaccharides, and monosaccharides are bonded to each other, which is characterized by glucoside bonding.
Glucose, a representative of monosaccharides, is a cellulosic block and is characterized by maintaining rigidity at the cell wall in plants.
Polysaccharides are characterized by being able to contain cellular molecules.
I will explain by moving to energy and metabolism.
Cells are life-active through energy, so organisms can be considered to belong to thermodynamics, and the energy system's crystal is composed of enthalpy and entropy.
Entropy is the flow of energy in disorder, and enthalpy is the amount of intrinsic energy.
In order to be more practical in G=H-TS, it is characterized by calculating by changing the formula to dG=dH-TdS.
Biochemistry can measure the free energy, enthalpy and entropy of the system.
H(after)-H(before)=dH<0 heat dissipation at date and dH>0 heat absorption at date and entropy is also described as S(after)-S(before)=dS
dG= G(product)-G(reatatnts) <0 Heat dissipates at a time. To illustrate this briefly, the cup is used as an example.
Compared to the unbreakable state of the cup, it is explained in many books.
The unbreakable state is called the reactant, and the broken state is called the product. The dG<0 is called spontaneous reaction, and the spontaneous reaction is discussed in the second law of thermodynamics, which changes from hot to cold.
This means that the enthalpy is lowered and the entropy is increased.
The time from A to B is called involuntary, energy absorption, and the time from B to A is called voluntary, energy release.
There are many students who are mistaken here, but increasing entropy does not affect the reaction speed.
Molecular concentration, temperature, catalyst, etc., affect the reaction rate.
If dG=0, the speed is the same and there is no change.
Living organisms react involuntarily in these reactions and gain energy by reversing entropy.
Therefore, I would like to mention that this phenomenon maintains order while creating disorder.

As an example, photosynthesis, which is an involuntary reaction, has a high oxidation like CO2, a low energy that is not reduced, a low oxidation like CH4, and a high energy when reduction is high, so it is good to know properly.
You can read the text to translated in koeran. : https://biostudy.tistory.com/6
'과학 > 생화학' 카테고리의 다른 글
| Origin of biochemistry and bioevolution and characteristics of water (0) | 2020.07.12 |
|---|---|
| Introduction to biochemistry, common sense needed (0) | 2020.07.12 |
| 술로 인한 숙취를 막는 물의 원리 (2) | 2016.05.19 |
| 탄수화물의 구성요소들과 예시들 -2- (0) | 2016.04.29 |
| 탄수화물의 구성요소들과 예시들 -1- (0) | 2016.04.28 |