Replication in cells (DNA, RNA) is a universal property. It is called inheritance and is called evolution to a large extent.
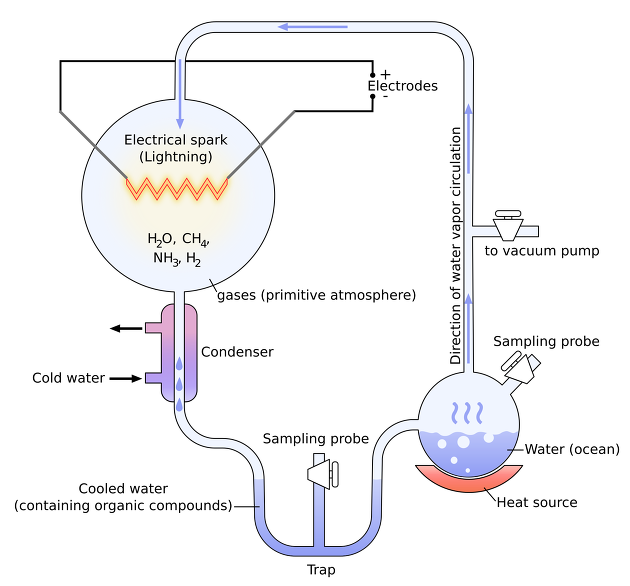
Miller's experiment was an experiment that imitated the appearance of a primitive Earth and put H2, H2O, CH4, NH3, etc. into an electric shock.
Through the above experiment, the amino acid, which is the origin of life, was obtained. In another experiment, +charged mud (cationic minerals are abundant + +charged) and-charged monomers are attached to Ploymers. You can see that the nucleotide is polymerized
It has been said that RNA has evolved more and life has evolved by delivering catalytic functions to proteins and information transfer functions to DNA.

When entering the aqueous science, 60% of our body is filled with water, and the water is polar, so most of the molecules with molecular weight similar to water with a molecular weight of 18 have the form of gas and gas, which has high cohesion and surface tension. Because of hydrogen bonding.
In addition, when the water is solid ice, it turns into a hexagonal structure.
Water forms 104.5 degrees through a bond between oxygen and hydrogen and a non-covalent electron pair.
For bonding, the bonding force is in the order of covalent bonding, ionic bonding, and hydrogen bonding.
In hydrogen bonding, C has 2.55, F 3.98, H 2.20, N 3.04, and O 3.44, so F>O>N>C>N.
In addition, each A, T bond and C, G bond that hydrogen bonds in the nucleic acid form two hydrogen bonds of A and T, and three bonds of C and G.
Water is also a solvent that dissolves many compounds, because water has a high electrostatic attraction, so it can dissolve either polar or ionic due to its large interaction, for example, glucose, Na, and Cl.
Most hydrocarbons do not have polar groups, so they are hydrophobic. To mix oil and water, they must be mixed by heat or stirring.
Therefore, hydrogen bonds are destroyed and dissolved through enthalpy.
Therefore, if a nonpolar compound is added to water, it will have a layer.

As an example of this, you can see a minority phenomenon. The picture above is Michelle.
The minority phenomenon is not a manpower, you can think of water molecules as being pushed, and lipids become structures.
In addition, some have a vesicle structure due to the membrane phenomenon of a double layer of phospholipids.
This prevents spontaneous spreading and can retain sodium and salt outside and potassium inside.
You can read the text to translated in korean : https://biostudy.tistory.com/7
'과학 > 생화학' 카테고리의 다른 글
| Biochemistry, simple biomolecules and energy metabolism (0) | 2020.07.12 |
|---|---|
| Introduction to biochemistry, common sense needed (0) | 2020.07.12 |
| 술로 인한 숙취를 막는 물의 원리 (2) | 2016.05.19 |
| 탄수화물의 구성요소들과 예시들 -2- (0) | 2016.04.29 |
| 탄수화물의 구성요소들과 예시들 -1- (0) | 2016.04.28 |